- Via Crocefisso 5, Milano, MI
- +(39) 02 99269173
- info@tenagro.com
Pure Colostrum is the result of state of the art research in collaboration with private and academic research centers. Tenagro has developed an innovative process technology and set-up a sophisticated analytical platform that together with a supply chain providing a high quality raw material, has allowed the development of a high quality and well characterized colostrum powder
Colostrum’s fully validated and controlled supply chain represents the first strong point in the process. Tenagro performs a direct evaluation and validation of each raw material supplier; the farming operation regarding colostrum’s collection and storage has been standardized; an effective, continuous, direct and rigorous control of the suppliers is applied.
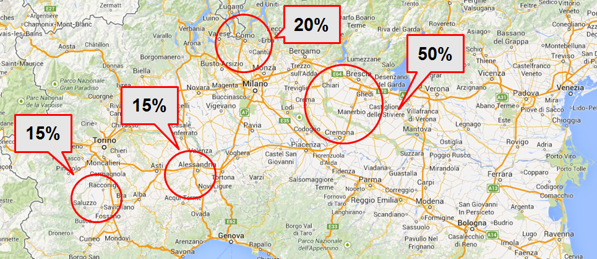
Colostrum is collected only from selected Italian herds and only from first milking (within 6 hours of the birth) to obtain the maximum content of active factors.After collection from the cow, the colostrum is kept refrigerated at -20°C until the industrial process begins.Microbiological and quality control analyses are carried out before starting the industrial process in order to select only high quality raw material.
Ensure raw material safety.
Guarantee high hygiene standard.
Minimize BSE risk.
Minimize zoonosis risk.
Minimize the decay of active factors content.
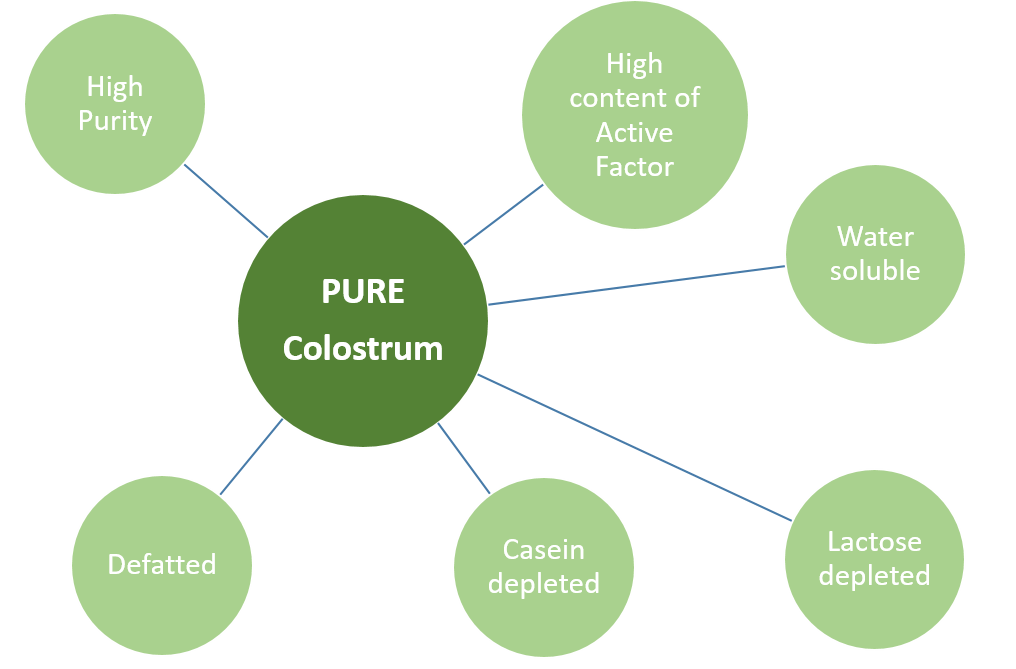
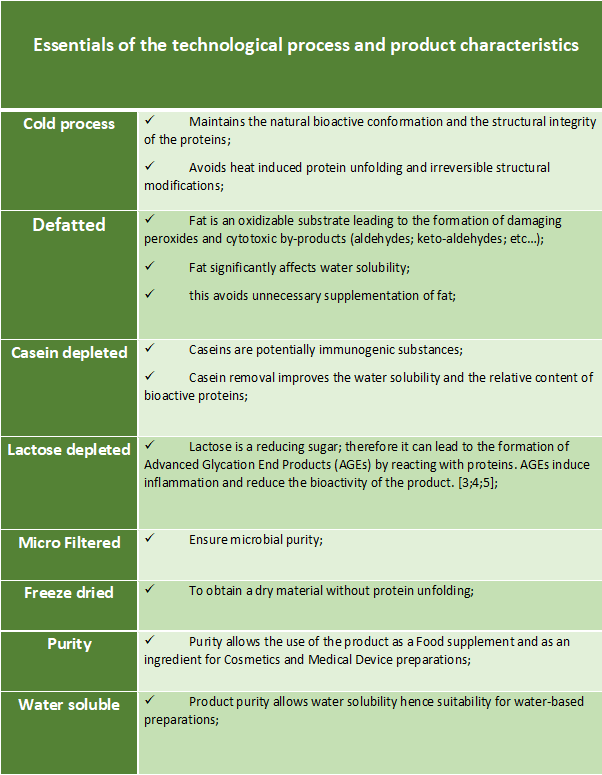
The colostrum purification is based on a technological process that allows the transformation of colostrum into a high quality powder, completely water soluble and devoid of fat, immunogenic caseins and reducing sugars that can affect the chemical and biological stability of the components and the safety of the product. Thanks to this process, the bioactivity of the colostrum components is maintained in an environment free from microbial contamination. The technological process is patent pending.
Tenagro’s control process is based on relevant legislation, it is weighted to the desired quality requirements and developed on the basis of a risk analysis approach.
Purification by proceduralized and validated processes, is performed in GMP and in food hygiene-compliant plants as the minimum requirement.
In process controls are performed to ensure the compliance of the product with the quality standard.
Tenagro’s colostrum extract is characterized by a high degree of purity to ensure the highest level of safety, the highest efficacy of the natural bioactive factors, the usability as a food and medical supplement and as a medical device ingredient.
Product is compliant with the following regulations: EU Reg. no. (EC) 853/2004; EU Reg. no. (EC) 1662/2006; EU Reg. no. (EC) 852/2004; EU Reg. no. (EC) 2377/90; EU Reg. no. (EC) 1881/2006; EU Reg. no. (EC) 2073/2005; EU Reg. no. (EC) 396/2005 .
© 2018 Tenagro | Powered by ADHOC SOLUTIONS